和誉医药五项研究成果精彩亮相2023AACR年会
和誉医药(港交所代码:02256)在2023年美国癌症研究协会(AACR)年会上公布了五项最新临床前研究结果。
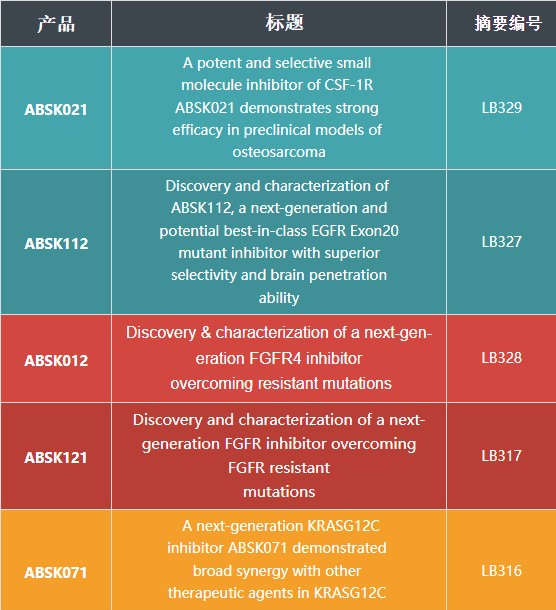
其中包括其自主研发的已获中美双突破性疗法认定的CSF-1R抑制剂Pimicotinib(ABSK021)和可能成为best-in-class 的新一代克服耐药突变的EGFR Exon20抑制剂ABSK112,以及新一代克服耐药突变的FGFR4 抑制剂ABSK012和FGFR抑制剂ABSK121,新一代KRAS抑制剂ABSK071的最新临床前和转化医学研究进展。
和誉医药在本届AACR年会上展示了以下壁报:
标题:
A potent and selective small molecule inhibitor of CSF-1R ABSK021 demonstrates strong efficacy in preclinical models of osteosarcoma
CSF-1R强效选择性小分子抑制剂ABSK021在临床前骨肉瘤模型中显示出强大的药效
摘要编号: LB329
研究背景:
Osteosarcoma is the most common primary malignant bone tumor in children and young adults. Surgery combined with multimodal chemotherapy remains as the standard treatment for osteosarcoma patients. However, patients with osteosarcoma metastasis have a five-year survival rate of less than 30%, and their long-term outcomes have not improved over the last 30 years, representing a high unmet medical need. CSF-1/CSF-1R signaling is crucial for the survival, function, proliferation and differentiation of myeloid lineage cells, including osteoclasts and monocytes/macrophages. Targeting CSF-1R either on tumor cells or tumor-associated macrophages has been reported to limit osteosarcoma progression in preclinical models. ABSK021, an oral, highly potent and selective small molecule inhibitor of CSF-1R, showed significant anti-tumor activity and favorable safety profile in patients with advanced tenosynovial giant cell tumor in phase 1b trial. We demonstrated the treatment potential of ABSK021 for osteosarcoma patients through a series of preclinical in vitro and in vivo experiments, as well as human osteosarcoma profiling.
骨肉瘤是儿童和年轻人中最常见的原发性恶性骨肿瘤。目前手术联合多种化疗是骨肉瘤患者的标准治疗方法。然而,骨肉瘤转移患者的五年生存率不到 30%,在过去 30 年中长期预后也未得到改善,这是一个高度未被满足的医疗需求。CSF-1/CSF-1R信号通路对于髓系细胞,包括破骨细胞和单核/巨噬细胞的存活、功能、增殖和分化至关重要。据报道,在临床前模型中,靶向肿瘤细胞或者肿瘤相关巨噬细胞上的CSF-1R可以限制骨肉瘤的进展。ABSK021是一个口服,高活性和高选择性的CSF-1R小分子抑制剂,在临床1b试验中对晚期腱鞘巨细胞瘤患者展现出显著的抗肿瘤疗效和良好的安全性。通过一系列临床前体外和体内实验,以及骨肉瘤患者的表达分析,我们证明了ABSK021具有治疗骨肉瘤患者的潜力。
结论:
ABSK021 has strong inhibition of CSF-1R activity and corresponding anti-tumor activity in preclinical osteosarcoma models. High prevalence of CSF-1R expression was also found in osteosarcoma patients, suggesting great potential of utilizing ABSK021 as a novel therapy to treat osteosarcoma patients in clinic.
ABSK021在临床前骨肉瘤模型中展现出强效的CSF1R活性抑制和相应的抗肿瘤活性。在骨肉瘤患者中也发现了高比例的CSF1R表达,表明ABSK021具有在临床上成为骨肉瘤新的治疗方法的巨大潜力。
标题:
ABSK112, a potential best-in-class EGFR exon 20 mutant Inhibitor with excellent selectivity and brain penetration
潜在最优EGFR exon20突变的小分子抑制剂ABSK112, 具有极佳选择性和入脑特性
摘要编号: LB327
研究背景:
EGFR Exon20 mutations are clinically validated oncogenic alterations including a wide spectrum of mutations occurring in lung cancer and various other cancer types. Although several EGFR Exon20 inhibitors have reached clinical stage or received approval, there still leave large room for improvement in safety and efficacy, likely due to their limited selectivity against wild-type EGFR or other kinases, suboptimal mutation coverage, and lack brain penetrating ability. Herein, we have discovered a novel and next-generation EGFR Exon20 mutation inhibitor, ABSK112. It showed high selectivity over wild-type EGFR and other kinases, as well as a more comprehensive coverage over majority of EGFR Exon20 mutations in comparison with other EGFR Exon20 inhibitors.
EGFR外显子20插入突变包括了一系列突变,是临床上确证的致癌性突变,在肺癌和其他多种肿瘤中均有发生。尽管已经有一些EGFR 外显子20插入突变抑制剂进入临床阶段或取得的临床批件,但由于诸如针对野生型EGFR或其他激酶的选择性有限,突变覆盖不完全,以及缺乏入脑特性等问题,在安全性和有效性上还留有很大进步空间。基于此,我们开发了全新的下一代EGFR外显子20插入突变抑制剂ABSK112。与其他同类抑制剂相比,它表现出针对野生型EGFR和其他激酶的优秀选择性,同时能够覆盖绝大部分 EGFR外显子20插入突变谱,并且有很好的入脑特性。
结论:
ABSK112 is a leading next-generation EGFR Exon20ins inhibitor with improved selectivity over wild-type EGFR and strong brain penetrating ability. It shows superior in vivo efficacy in various EGFR Exon20ins xenograft models, and broader spectrum of mutation coverage than clinical stage competitors.
ABSK112是领先的新一代EGFR外显子20插入突变抑制剂,具有针对比野生型EGFR的高选择性和高脑穿透能力,并且在各种EGFR外显子20插入突变的异种移植模型中具有出色的体内功效,比临床阶段竞争对手具有更广泛的突变覆盖范围。
标题:
Discovery & characterization of a next-generation FGFR4 inhibitor overcoming resistant mutations
可以克服耐药突变的新一代FGFR4抑制剂的发现和表征
摘要编号: LB328
研究背景:
Aberrant activation of FGF19-FGFR4 signaling pathway plays an essential role in the tumorigenesis of Hepatocellular carcinoma (HCC) and FGFR4 inhibitors have shown preliminary efficacy in recent clinical trials for patients with FGF19 overexpression. However, the observed responses only lasted a few months before tumors relapse. Acquired FGFR4 resistant mutations were found in ~30% of FGFR4 inhibitor responsive patients. Similar FGFR4 mutations haven also been found de novo in about 7-10% of Rhabdomyosarcoma (RMS) and ER-treated invasive lobular carcinoma patients. First generation FGFR4 inhibitors have minimal activity against these de novo or acquired resistant mutations. Therefore, next-generation of FGFR4 inhibitors are needed to overcome these resistant FGFR4 mutations to provide better treatment options for patients. Using advanced computation-aided structural analysis and medicinal chemistry design, we have discovered a next-generation small molecule FGFR4 inhibitor, ABSK012, and demonstrated its strong activities against de novo and acquired resistant FGFR4 mutations while retaining inhibition for wild-type FGFR4.
成纤维细胞生长因子(FGF)19和成纤维细胞生长因子受体(FGFR)4信号通路的过度激活在肝细胞癌(HCC)的癌症发生过程中扮演着非常重要的角色,FGFR4抑制剂也已经在临床实验中显示出对于FGF19过表达病人的药效。然而,所观察到的临床活性经常在几个月后就发生了肿瘤的复发。在大约30%的FGFR4抑制剂有效的病人中都会有获得性的FGFR4耐药突变的发生。类似的FGFR4突变也被发现在7-10%的横纹肌肉瘤(RMS)以及雌激素受体阳性的侵入性的小叶乳腺癌病人中。一代的FGFR4抑制剂对于这些获得性或者新生的耐药突变几乎没有活性。因此,需要新一代的FGFR4抑制剂为病人提供更好的医疗方案。采用计算机辅助结构设计以及药物化学的设计,我们发现了一个新一代的FGFR4抑制剂,ABSK012.它在新生及获得性的FGFR4耐药突变中展现了很强的活性,且对野生型的FGFR4也保留了很强的抑制。
结论:
ABSK012, presented here by Abbisko Therapeutics, is a highly potent, selective, and next-generation small molecule FGFR4 inhibitor overcoming FGFR4 mutations resistant to first-generation inhibitors. Its superior preclinical profile supports its fast-track development into clinic.
这些数据首次证明了ABSK012是新一代的高选择性的强效小分子 FGFR4 抑制剂,而且可以克服FGFR4的耐药突变。它优秀的临床前性质支持它尽快进入临床开发阶段进行评估。
标题:
Discovery and characterization of a next-generation FGFR inhibitor overcoming FGFR resistant mutations
可以克服耐药突变的新一代FGFR抑制剂的发现和表征
摘要编号: LB317
研究背景:
FGFRs play important roles in cancer development and inhibition of FGFR could disrupt tumor cell proliferation and growth. Four selective FGFR inhibitors have been approved (erdafitinib, pemigatinib, infigratinib, and futibatinib) and several others are in clinical development. Unfortunately upon treatment with these first-generation FGFR inhibitors, acquired resistance often develops and is frequently associated with the emergence of secondary FGFR2/3 kinase domain mutations. Therefore, selectively targeting FGFR2/3 as well as their resistant mutations may render a second-generation treatment approach for the refractory/relapsed patients. Using advanced computation-aided structural analysis and medicinal chemistry design, we have discovered a novel, next-generation, and highly selective FGFR inhibitor, ABSK121. This novel inhibitor demonstrated robust anti-tumor activity in FGFR-dependent tumor models with strong activities against not only de novo but also acquired resistant mutations.
成纤维细胞生长因子受体(FGFR)在肿瘤的发展中有重要的作用,抑制FGFR可以阻碍肿瘤细胞的增殖和生长。有四个选择性的FGFR抑制剂被批准上市(erdafitinib, pemigatinib, infigratinib, 以及 futibatinib),还有一些其他在临床开发阶段。不幸的是,在这些一代的FGFR抑制剂治疗之后,经常会产生获得性的耐药,而且常常和次生的FGFR2/3激酶区域突变的发生相关。因此选择性的靶向FGFR2/3以及它们的耐药突变,可以为复发的病人提供二代的治疗手段。采用计算机辅助结构设计以及药物化学的设计,我们发现了一个新的下一代的选择性FGFR抑制剂,ABSK121。它在FGFR依赖性的肿瘤模型中展现了非常好的抗肿瘤活性,而且对新生及获得性的FGFR耐药突变都有很强的活性。在本次壁报中,我们展示了ABSK121的临床前体外和体内数据。
结论:
ABSK121, presented here by Abbisko Therapeutics, is a highly potent, selective, and next-generation small molecule FGFR inhibitor with great potency against resistant FGFR mutations. Its superior profile supports fast-track preclinical and clinical development.
这些数据首次证明了ABSK121是新一代的高选择性的强效小分子 FGFR抑制剂,而且可以克服FGFR的耐药突变。它优秀的临床前性质支持它尽快进入临床开发阶段进行评估。
标题:
A next-generation KRASG12C inhibitor ABSK071 demonstrated broad synergy with other therapeutic agents in KRASG12C mutated cancer models
新一代KRAS G12C抑制剂ABSK071在KRAS G12C突变肿瘤模型中与其他药物联用表现出广泛的联合用药效果摘要编号: LB316
研究背景:
KRAS is frequently mutated in human cancers, including pancreatic (~90%), colorectal (~35%), and lung cancer (~25%). The KRASG12C mutation (single amino acid substitution of cysteine for glycine at position 12) accounts for ~14% of lung cancer, ~4% of colorectal cancer, and ~2% of pancreatic cancer. Currently, two covalent KRASG12C inhibitors, namely sotorasib (AMG-510) and adagrasib (MRTX-849), have been approved as monotherapy to treat locally advanced or metastatic NSCLC with KRASG12C mutation through accelerated approval process.
Despite the beneficial effects of KRASG12C inhibitors in clinic for certain patients, the limited antitumor efficacy in most patients and potential drug resistance are major concerns. A next-generation inhibitor with better inhibitory activity may improve anti-tumor efficacy. Combination with other therapeutic agents may also improve the single-agent activity of KRASG12C inhibitors. These approaches could both overcome the limitations of sotorasib and adagrasib and provide additional benefits to patients.
KRAS基因在人类肿瘤中经常发生突变,在约90%的胰腺癌、约35%的结直肠癌和约25%肺癌病人中会发生KRAS基因的突变。KRAS G12C突变(第12位的甘氨酸突变为半胱氨酸)约占肺癌的14%,结直肠癌的4%,以及胰腺癌的2%。目前,已有两种KRAS G12C共价抑制剂通过FDA的加速审批程序被批准上市,单药用以治疗伴有KRAS G12C突变的局部晚期或转移性非小细胞肺癌(NSCLC),它们分别为安进公司的Sotorasib (AMG-510)和Mirati公司的Adagrasib (MRTX-849)。
尽管使用KRAS G12C抑制剂的部分患者表现出一定的临床获益,但对大多数患者来说其抗肿瘤效果有限,并且存在潜在的耐药风险。因此,一方面需要开发抗肿瘤活性更好的新一代KRAS G12C抑制剂以提高抗肿瘤效果,另一方面需要通过与其他药物的联合用药来提高KRAS G12C抑制剂的抗肿瘤活性。这些方法可以克服Sotorasib和Adagrasib的局限性,并为患者带来更大的临床获益。
结论:
ABSK071 is a next-generation KRASG12C inhibitor with greater activity and anti-tumor efficacy in vitro and in vivo. It also demonstrated broad synergistic effects with a large set of targeted agents and immuno-oncology agents, indicating its strong potential in combinatory therapy in treating a wider range of KRASG12C-dependent cancers.
ABSK071是新一代KRAS G12C抑制剂,表现出更强的体外生物学活性以及体内抗肿瘤药效。ABSK071与多种靶向治疗药物和免疫治疗药物联用表现出广泛的联合用药效果,预示着其具有更大的联合用药潜力用以治疗更多的KRAS G12C突变的肿瘤患者。